Cancers, Vol. 12, Pages 3265: Patient-Reported Outcomes of Palbociclib Plus Exemestane with GnRH Agonist versus Capecitabine in Premenopausal Women with Hormone Receptor-Positive Metastatic Breast Cancer: A Prospective, Open-Label, Randomized Phase ll Trial (KCSG-BR 15-10)
Cancers doi: 10.3390/cancers12113265
Authors: Soohyeon Lee Seock-Ah Im Gun Min Kim Kyung Hae Jung Seok Yun Kang In Hae Park Jee Hyun Kim Kyoung Eun Lee Hee Kyung Ahn Moon Hee Lee Hee-Jun Kim Han Jo Kim Jong In Lee Su-Jin Koh Yeon Hee Park
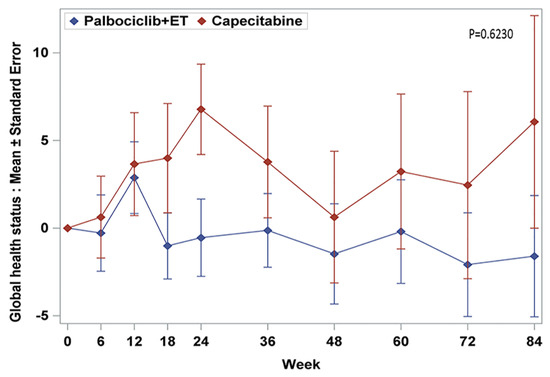
In the era of CDK4/6 inhibitors in hormone receptor (HR)-positive, HER2-negative metastatic breast cancer, few trials have been specifically studied to compare quality of life between palbociclib plus endocrine therapy (ET) and cytotoxic chemotherapy exclusively in premenopausal women. We aimed to evaluate differences of patient report outcomes (PROs) between palbociclib plus ET and capecitabine. PROs were assessed using EORTC QLQ-C30 at baseline, every 6 weeks, and the end of treatment. All EORTC QLQ-30 scores were maintained from baseline to the end of treatment. Patients treated palbociclib plus ET arm experienced delay in time-to-deterioration of physical functioning (HR = 0.58, 95% CI, 0.36 to 0.84, p = 0.0058), nausea and vomiting (HR = 0.48; 95% CI, 0.32 to 0.73, p = 0.0005), and diarrhea (HR = 0.42; 95% CI, 0.27 to 0.65, p = 0.001). There was a numeric trend for worsening of insomnia (HR = 1.43; 95% CI, 0.96 to 2.16, p = 0.079) and favoring of appetite loss (HR = 0.69, 95 % CI, 0.44 to 1.07, p = 0.09) in the palbociclib plus ET arm. Premenopausal patients with palbociclib plus ET maintained QoL without compromising treatment efficacy.


No comments:
Post a Comment